Topics and Pictures
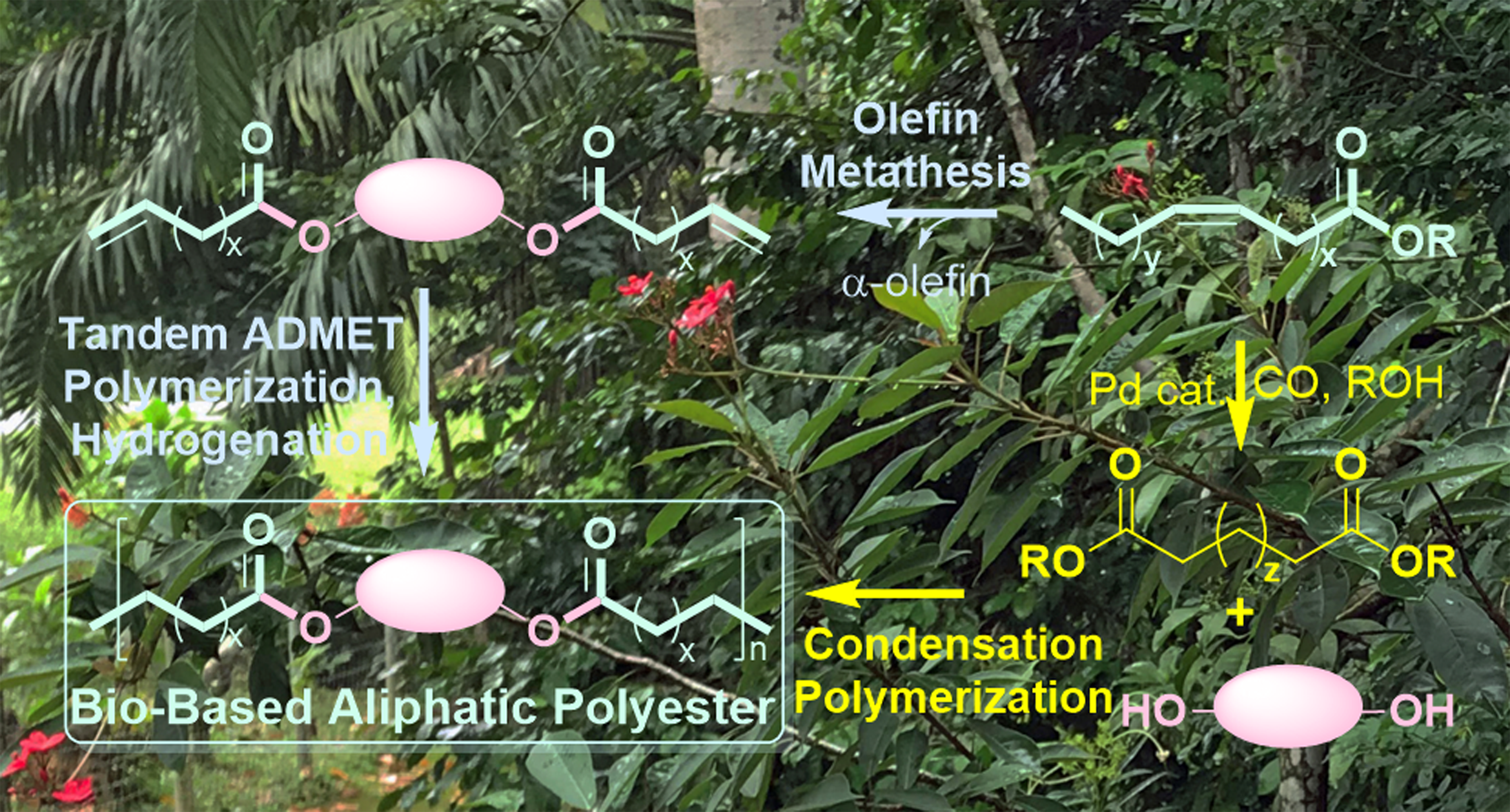
“Synthesis of high molecular weight biobased aliphatic polyesters by acyclic diene metathesis polymerization in ionic liquids”, ACS Omega, Here
February 2023 Bangkok, Thailand
“Synthesis of ethylene/isoprene copolymers containing cyclopentane/cyclohexane units as unique elastomers by half-titanocene catalysts”, Macromolecules, Here
“Propylene cyclic olefin copolymers with cyclopentene, cyclohexene, cyclooctene, tricyclo[6.2.1.0(2,7)]undeca-4-ene, and with tetracyclododecene: The synthesis and effect of cyclic structure on thermal properties”, Macromolecules Here
December 2022
E Asia Meeting in Philippines (White Beach, Boracay), Symposium on Degradable Polymers and Film Composites 2022 (Manila)

November 2022
Vidyasirimedhi Institute of Science and Technology (VISTEC), Thammasat University (Pattaya), 11th The International Symposium on Feedstock Recycling of Polymeric Materials (ISFR, Pattaya)

October 2022 (Nagano) The Japan Petroleum Institute (JPI)
September 2022 (Toyama) Catalysis Society of Japan

“Transesterification of methyl-10-undecenoate and poly (ethylene adipate) catalyzed by (cyclopentadienyl)titanium trichlorides as model chemical conversions of plant oils and acid-, base-free chemical recycling of aliphatic polyesters”, ACS Sustainable Chem. Eng., Here
“CaO catalyzed transesterification of ethyl 10-undecenoate as a model reaction for efficient conversion of plant oils and their application to depolymerization of aliphatic polyesters” ACS Sustainable Chem. Eng., Here
International Symposium on Homogeneous Catalysis (ISHC, Lisbon, July, 2022) Plenary Lecture; Visiting University of Hull, UK


Pure and Applied Chemistry International Conference 2022 (PACCON 2022, Bangkok, June, 2022)Visiting PPC, Chula

New laboratory picture (June, 2022)

“Effect of borate cocatalysts toward activity and comonomer incorporation in ethylene copolymerization by half-titanocene catalysts in methylcyclohexane”, ACS Org. Inorg. Au, Here
“La(iii)-Catalysed degradation of polyesters to monomers via transesterifications”, Chem. Commun. , Here
“Effect of phosphine, B(C6F5)3 in ring opening metathesis polymerization (ROMP) of cyclic olefins by (Arylimido)Vanadium(V)-Alkylidene Catalysts and the chain transfer ROMP of cycloheptene”, J. Jpn. Petrol. Inst. , Here
“High conversion of CaO-catalyzed transesterification of vegetable oils with ethanol”, J. Oleo Sci., Here
“Star-shaped ROMP polymers coated with oligothiophenes that exhibit unique emission”, ACS Omega, Here
March 2022 (Kochi, Matsuyama)

“Solution XAS analysis for reactions of phenoxide-modified (arylimido)vanadium(V) dichloride and (oxo)vanadium(V) complexes with Al alkyls: Effect of Al cocatalyst in ethylene (co)polymerization”, Catalysts, Here
“Analysis of ethylene copolymers with long chain α-olefins (1-dodecene, 1-tetradecene, 1-hexadecene): A transition between main chain crystallization and side chain crystallization”, ACS Omega, Here
“Transesterification of ethyl-10-undecenoate using Cu deposited V2O5 catalyst as a model reaction for efficient conversion of plant oils to monomers, fine chemicals”, ACS Omega, Here
November 2021 (Hakodate) Japan Petroleum Institute

New laboratory picture (November, 2021)

“Ethylene/myrcene copolymer as new bio-based elastomers prepared by coordination polymerization using titanium catalysts”, Macromolecules, Here
“Synthesis of semi-crystalline long chain aliphatic polyesters by ADMET copolymerization of dianhydro-D-glucityl bis(undec-10-enoate) with 1,9-decadiene and tandem hydrogenation”, Catalysts, Here
“Ethylene-norbornene-1-octene terpolymers with high 1-octene content, molar masses, tunable Tg values, in high yield by half-titanocene catalysts”, Polym. Chem., Here
“Vanadium(V) arylimido alkylidene N-heterocyclic carbene alkyl and perhalophenoxy alkylidenes for the cis, syndiospecific ring opening metathesis polymerization of norbornene”, Organometallics, Here
“Effect of para-substituents in ethylene copolymerizations with 1-decene, 1-dodecene, and with 2-methyl-1-pentene using phenoxide modified half-titanocenes–MAO catalyst systems”, Chem. Open, Here
“Ring opening metathesis polymerization (ROMP) of norbornenes by
(arylimido)niobium(V)-alkylidene catalysts, Nb(CHSiMe3)(NAr)[OC(CF3)3](PMe3)2”, J. Jpn. Petrol. Inst., Here
“Synthesis of bio-based aliphatic polyesters from plant oils by efficient molecular catalysis: A selected survey from recent reports”, ACS Sustainable Chem. Eng., Here Cover
“Ethylene copolymerization with limonene, β-pinene: New bio-based polyolefins prepared by coordination polymerization” Macromolecules, Here
New laboratory picture (April, 2021)

March 2021 (Izumo, Shimane)

“Theoretical study of reaction mechanism for half-titanocene-catalyzed styrene polymerization, ethylene polymerization, and styrene-ethylene copolymerization: Roles of the neutral Ti(III) and the cationic Ti(IV) species” Organometallics, Here
“Recent developments in Z-selective olefin metathesis reactions by molybdenum, tungsten, ruthenium, and vanadium catalysts”
Adv. Synth. Catal. (Introduced as VIP, Very Important Paper),
Backside Cover Article / The cover is Here.) https://doi.org/10.1002/adsc.202001117
“Synthesis of amorphous ethylene copolymers with 2-vinylnaphthalene, 4-vinylbiphenyl and 1-(4-vinylphenyl)naphthalene”
Macromolecules https://doi.org/10.1021/acs.macromol.0c02224
New laboratory picture (January, 2021)

“Observation of intramolecular interaction in fluorescent star-shaped polymers: Evidence for energy hopping between branch chains”
J. Phys. Chem. B Here
“Vanadium catalysed olefin metathesis and related chemistry”
Vanadium Catalysis (Book Chapter) https://doi.org/10.1039/9781839160882-00417
“Norbornene-functionalized plant oils for bio-based thermoset films and binders of silicon-graphite composite electrodes”
ACS Omega https://doi.org/10.1021/acsomega.0c02645 (Open Access)
November 2020 (Kumamoto) Japan Petroleum Institute

“Synthesis, structural analysis of four coordinate (arylimido)Niobium(V) dimethyl complexes containing phenoxide ligand: MAO-Free ethylene polymerization by the cationic Nb(V)–methyl complex”
Organometallics https://doi.org/10.1021/acs.organomet.0c00567
“Effect of SiMe3, SiEt3 para-substituents for exhibiting high activity, introduction of hydroxy group in ethylene copolymerization catalyzed by phenoxide-modified half-titanocenes”
Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202010559
September, 2020 (Hakone)

“The synthesis of cyclic olefin copolymers (COCs) by ethylene copolymerisations with cyclooctene, cycloheptene, and with tricyclo[6.2.1.0(2,7)]undeca-4-ene: Effect of cyclic monomer structures on thermal properties”
Polym. Chem. (Backside Cover Article / The cover is Here.) https://doi.org/10.1039/D0PY00940G
“Phenoxide-modified half-titanocenes supported on star-shaped ROMP polymers as efficient catalyst precursors for ethylene copolymerization”
Organometallics (Front Cover Article / The cover is Here., Editor invitation) https://doi.org/10.1021/acs.organomet.0c00365
August, 2020 Online seminar series in Bioenergy and Catalysis Research by Thammasat University (TU)

“Synthesis of bio-based long chain polyesters by acyclic diene metathesis (ADMET) polymerization and tandem hydrogenation, and depolymerization with ethylene”
ACS Omega https://doi.org/10.1021/acsomega.0c01965 (Open Access)
“Cis-Specific ring opening metathesis polymerisation (ROMP) of cyclic olefins by (pentafluorophenylimido)vanadium(V)-alkylidene,V(CHSiMe3)(NC6F5)[OC(CF3)3](PMe3)2”
Catal. Sci. Technol. (Inside Back Cover Article / The cover is Here.) https://doi.org/10.1039/D0CY00938E
“(Arylimido)niobium(V)-alkylidenes, Nb(CHSiMe3)(NAr)[OC(CF3)3](PMe3)2, that enable to proceed living metathesis polymerization of internal alkynes”
Macromolecules https://doi.org/10.1021/acs.macromol.0c00874
PACCON2020 (Bangkok, February 2020)

“Synthesis of ultrahigh molecular weight polymers containing reactive functionality with low PDIs by polymerizations of long-chain α-olefins in the presence of their nonconjugated dienes by Cp*TiMe2(O-2,6-iPr2C6H3)–borate catalyst”
Polymers (Special issue “Catalytic Polymerization” dedicated to Prof. Tritto.) https://doi.org/10.3390/polym12010003 (Open Access)
“Solution XANES and EXAFS analysis of active species of titanium, vanadium complex catalysts in ethylene polymerisation/dimerisation and syndiospecific styrene polymerisation”
Dalton Trans. (Perspective, Invited, Backside Cover / The cover is Here.) https://doi.org/10.1039/D0DT01139H
“Time-dependent DFT study of K-edge spectra for vanadium and titanium complexes: Effects of chloride ligands on pre-edge features”
Phys. Chem. Chem. Phys. https://doi.org/10.1039/C9CP05891E
January, 2020 Visiting Beijing (Institute of Chemistry, Chinese Academy of Sciences)
January, 2020 Visiting Manila, Philippines (Ateneo de Manila University and University of the Philippines, Diliman)

January, 2020 CU-TMU symposium (@TMU hosted by Prof. Shishido) Also see here (Petromat)

December, 2019 Visiting Bangkok (Chulalongkorn University, Mahidol University and Thammasat University)

December, 2019 Visiting UKM, Malaysia

December, 2019 (Singapore) 16th Pacific Polymer Conference (PPC16)
December, 2019 (Hiroshima) Asian Polyolefin Workshop2019 (APO2019)
“Solution X-ray absorption spectroscopy (XAS) for analysis of catalytically active species in reactions with ethylene by homogeneous (imido)vanadium(V) complexes – Al cocatalyst systems”
Catalysts (Feature Article by invitation) https://doi.org/10.3390/catal9121016 (Open Access)
“Solution XAS analysis for exploring active species in syndiospecific styrene polymerization and 1-hexene polymerization using half-titanocene – MAO catalysts: Significant changes in the oxidation state in the presence of styrene” Organometallics https://doi.org/10.1021/acs.organomet.9b00638
October, 2019 (Prof. K. Dawood, Cairo Univ. Egypt, JSPS program)

“XAS Analysis for reactions of (arylimido)vanadium(V) dichloride complexes containing anionic NHC that contains weakly coordinating B(C6F5)3 moiety (WCA-NHC) or phenoxide ligands with Al alkyls: A potential ethylene polymerization catalyst with WCA-NHC ligand” ACS Omega (invited) https://doi.org/10.1021/acsomega.9b02828 (Open Access)
September, 2019 (Nagasaki) Catalysis Society of Japan

“Direct observation of catalytically active species In reaction solution by X-ray absorption spectroscopy (XAS)” Jpn. J. Appl. Phys. (Mini review in the Special issue, invited) https://iopscience.iop.org/article/10.7567/1347-4065/ab3e5c (Open Access)
August, 2019 (The 8th Asia-Pacific Congress on Catalysis, APCAT8, Bangkok, Thailand)
July, 2019 (7th international symposium of Institute for Catalysis in Hokkaido University, Sapporo)
“Synthesis of half-titanocenes containing anionic N-heterocyclic carbenes that contain a weakly coordinating borate moiety (WCA-NHC), Cp’TiX2(WCA-NHC), and their use as catalysts for ethylene (co)polymerization” Organometallics https://doi.org/10.1021/acs.organomet.8b00841
“Well-defined end-functionalized conjugated polymers/oligomers exhibiting unique emission properties through the end-groups: The exclusive synthesis by combined olefin metathesis with Wittig-type coupling” Macromol. Mater. Eng. (Feature article in the Special issue, invited) https://doi.org/10.1002/mame.201900307
“(Arylimido)vanadium(V)-alkylidene complexes as catalysts for ring-opening metathesis polymerization (ROMP) of cyclic olefins: Ligand design for exhibiting the high activity” Chin. J. Polym. Sci. (Feature article in the Special issue, invited) https://link.springer.com/article/10.1007/s10118-019-2298-9
July, 2019 (23rd International Symposium on Olefin Metathesis and Related Chemistry, ISOM23, Barcelona, Spain)

June, 2019 (5th Blue Sky Conference on Catalytic Olefin Polymerization, Napoli and Sorrento, Italy)

June, 2019 (2019 US-Japan Polymer Symposium, Stanford, CA, USA)

“Effect of supported MAO cocatalysts in ethylene polymerization and ethylene/1-hexene copolymerization using Cp*TiCl2(O-2,6-iPr2C6H3) catalyst” Molecular Catalysis (Special issue, invited) https://doi.org/10.1016/j.mcat.2019.110490
“Synthesis of ultrahigh molecular weight polymers with low PDIs by polymerizations of 1-decene, 1-dodecene, and 1-tetradecene by Cp*TiMe2(O-2,6-iPr2C6H3)–Borate Catalyst” Molecules (Special issue, invited) https://www.mdpi.com/1420-3049/24/8/1634 (Open Access)
New laboratory picture (June, 2019)

June, 2019 (102nd Canadian Chemistry Conference and Exhibition, Quebec, Canada)

April, 2019 (Prof. Fujiki retirement party @Tokyo)

“Synthesis of new polyesters by acyclic diene metathesis polymerization of bio-based α,ω-dienes prepared from eugenol and castor oil (undecenoate)” RSC Adv. https://pubs.rsc.org/en/content/articlehtml/2019/ra/c9ra01065c (Open Access)
“(Arylimido)niobium(V) complexes containing 2-pyridylmethylanilido ligand as catalyst precursors for ethylene dimerization that proceeds via cationic Nb(V) species” Organometallics https://doi.org/10.1021/acs.organomet.9b00017 (Open Access)
“Reactions of (arylimido)vanadium(V)-trialkyl complexes with phenols: Effects of arylimido ligands and phenols for formation of the vanadium phenoxides” ACS Omega https://www.mdpi.com/2073-4344/8/12/670 (Open Access)
“Dialkylaluminum 2-substituted 6,6-dimethylcyclopentylpyridin-7-oxylates toward structural-differentiating the ring opening polymerization of ɛ-caprolactone and L-lactides” Dalton Trans. (Backside Cover) DOI:10.1039/C9DT00137A
February, 2019 (3D Active Site Science Symposium in London)

January, 2019 (Sumo tournament with Prof. Peter Junk, James Cook Univ.)

“Synthesis of soluble star-shaped polymers via in and out approach by ring-opening metathesis polymerization (ROMP) of norbornene: Factors affecting the precise synthesis” Catalysts https://www.mdpi.com/2073-4344/8/12/670 (Open Access)
December, 2018 (C&FC2018, Bangkok) http://www.cfc2018.org/

“(Imido)vanadium-alkylidene complex catalysts for efficient ring-opening metathesis polymerization of cyclic olefins” Kobunshi Ronbunshu (Special issue, Accounts) https://doi.org/10.1295/koron.2018-0034
November, 2018 (IKCOC2018, Kyoto)

October-November, 2018: Visiting Université Libre de Bruxelles (ULB), University of Stuttgart, Technische Universität Braunschweig
“Facile in situ generation of highly active (arylimido)vanadium(V)-alkylidene catalysts for ring-opening metathesis polymerization (ROMP) of cyclic olefins by immediate phenoxy ligand exchange” Chem. Commun. https://pubs.rsc.org/en/Content/ArticleLanding/2018/CC/C8CC07974A#!divAbstract
October, 2018 (HTPM-X, Beijing)

“Efficient conversion of renewable unsaturated fatty acid methyl esters by cross metathesis with eugenol” ACS Omega https://pubs.acs.org/doi/10.1021/acsomega.8b01695 (Open Access)
September, 2018 (Hakodate) Catalysis Society of Japan

Department of Chemistry, Thammasat University (Bangkok, Thailand, September, 2018)

“One-pot synthesis of end-functionalised soluble star-shaped polymers by living ring-opening metathesis polymerisation using molybdenum-alkylidene catalyst”
RSC Adv. https://pubs.rsc.org/en/content/articlelanding/2018/ra/c8ra05229h#!divAbstract (Open Access)
“Interaction between the end groups and the main chain of conjugated polymers by time-resolved EPR and fluorescence spectroscopy” Molecular Physics (special issue) https://www.tandfonline.com/doi/abs/10.1080/00268976.2018.1510140?journalCode=tmph20
July, 2018 (GT2018, Beijing, China)

“(Arylimido)vanadium(V)-alkylidenes containing chlorinated phenoxy ligands: Thermally robust, highly active catalyst in ring-opening metathesis polymerization of cyclic olefins” Organometallics https://pubs.acs.org/doi/10.1021/acs.organomet.8b00231
“Synthesis of (arylmido)niobium(V) complexes containing ketimide, phenoxide ligands, and some reactions with phenols, alcohols”
ACS Omega https://pubs.acs.org/doi/abs/10.1021/acsomega.8b01065 (Open Access)
“Solution XAS analysis of various (imido)vanadium(V) dichloride complexes containing monodentate anionic ancillary donor ligands: Effect of aluminium cocatalyst in ethylene/norbornene (co)polymerization” J. Jpn. Petrol. Inst. (special issue, invited) https://www.jstage.jst.go.jp/article/jpi/61/5/61_282/_pdf/-char/ja (Open Access)
June, 2018 (PC2018, Changchun, China)

New laboratory picture (June, 2018)

“Terthiophene functionalized conjugated triarm polymers containing poly(fluorene-2,7-Vinylene) arms having different cores. Synthesis and their unique optical properties” ACS Omega https://pubs.acs.org/doi/10.1021/acsomega.8b00676 (Open Access)
“Solution XAS analysis for exploring the active species in homogeneous vanadium complex catalysis” J. Phys. Soc. Jpn. https://journals.jps.jp/doi/abs/10.7566/JPSJ.87.061014 (Open Access)
“Noticeable chiral center dependence of signs and magnitudes in circular dichroism (CD) and circularly polarized luminescence (CPL) spectra of all-trans poly(9,9-dialkyl-fluorene-2,7-vinylene)s bearing chiral alkyl side chains in solution, aggregates, and in thin film”
Macromolecules Here
“Olefin polymerization by vanadium complex catalysts”, Handbook of Transition Metal Polymerization Catalysts (2nd Ed.), Ray Hoff (Ed.), Wiley, 313-338 (2018) https://onlinelibrary.wiley.com/doi/book/10.1002/9781119242277
February: Our effort was introduced in Tutorial, “Activation of Carbon–Hydrogen Bonds via 1,2-RH-Addition/-Elimination to Early Transition Metal Imides (by Prof. P. T. Wolczanski)”
Organometallics Here 1,2-C-H Bond activation of benzene by vanadium-alkylidene: here
January 30 (TMU): TMU and PetroMat Joint Mini Symposium on Catalysis and Advanced Materials 2018

“Ethylene copolymerization with 4-methylcyclohexene or 1-methylcyclopentene by half-titanocene catalysts: Effect of ligands and microstructural analysis of the copolymers” Macromolecules http://doi: 10.1021/acs.macromol.7b02484
“Olefin metathesis polymerization: Some recent developments in the precise polymerizations for synthesis of advanced materials (by ROMP, ADMET)” Tetrahedron (report) https://doi.org/10.1016/j.tet.2017.12.041
Hefei&Beijing (Hefei University of Technology, University of Science and Technology of China)

“Synthesis, structural analysis of (imido)vanadium dichloride complexes containing 2-(2’-benz-imidazolyl)pyridine ligands: Effect of Al cocatalyst for efficient ethylene (co)polymerization” ACS Omega http://doi.org/10.1021/acsomega.7b01225
“Vanadium NMR chemical shifts of (imido)Vanadium(V) dichloride complexes with imidazolin-2-iminato and imidazolidin-2-iminato ligands: A cooperation with quantum-chemical calculations and multiple linear regression analyses” J. Phys. Chem. A http://pubsdc3.acs.org/doi/suppl/10.1021/acs.jpca.7b08328
“Synthesis and reaction chemistry of alkylidene complexes with titanium, zirconium, vanadium, and niobium: Effective catalysts for olefin metathesis polymerization and the other organic transformations” Adv. Organomet. Chem. https://www.sciencedirect.com/science/article/pii/S0065305517300205
November-December, 2017 (ULB, Brussels)

October, 2017 (APO2017, Tianjin; ICCAS, Beijing)

“Cis Specific chain transfer ring-opening metathesis polymerization using a vanadium(V)-alkylidene catalyst for efficient synthesis of end-functionalized polymers” Organometallics http://pubs.acs.org/doi/10.1021/acs.organomet.7b00675
September, 2017 (Hohhot, Inner Mongolia University)

“Effects of terthiophene as the end-groups in triblock copolymers consisting of poly(fluorene vinylene) and oligo(phenylene vinylene): Time-resolved fluorescence and its anisotropy” J. Photochem. Photobiol. A: Chem.
“Effect of Al cocatalyst in ethylene and ethylene/norbornene (Co)polymerization by (imido)vanadium dichloride complexes containing anionic N-heterocyclic carbenes having weakly coordinating borate moiety” J. Jpn. Petrol. Inst. (special issue, invited) https://doi.org/10.1627/jpi.60.256 (Open Access)
August-October, 2017 (Institute of Chemistry, Chinese Academy of Sciences, Beijing)

“Synthesis and structural analysis of palladium(II) complexes containing neutral or anionic C2-symmetric bis(oxazoline) ligands: Effects of substituents in the 5 position” ACS Omega http://doi.org/10.1021/acsomega.7b00457 (Open Access)
July, 2017 (Bangkok, Thailand)
July, 2017 (Beijing, China): Intensive lecture course in University of Chinese Academy of Sciences

“Facile, Efficient Synthesis of Star-Shaped π-Conjugated Systems by Combined Olefin Metathesis with Wittig-type Coupling”
J. Chin. Chem. Soc. (special issue, C&FC2016)
http://onlinelibrary.wiley.com/doi/10.1002/jccs.201700058/full
Highlight in Olefin Metathesis — Fundamentals and Frontiers: (imido)vanadium-akylidene catalyst
Virtual Issue, Organometallics Here or Here
New laboratory picture (June, 2017)

“Ring opening metathesis polymerization of norbornene or tetracyclododecene with cyclooctene by using (arylmido)vanadium(V)-alkylidene catalyst”
J. Poly. Sci. PartA: Polymer Chem. (special issue for celebrating Prof. Grubbs 75th birthday)http://onlinelibrary.wiley.com/doi/10.1002/pola.28622/full
“Synthesis of poly(arylene vinylene)s containing different end groups by combined acyclic diene metathesis polymerization with Wittig-type coupling” Angew. Chem. Int. Ed.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201700466/full
Our paper accepted in Angew. Chem. Int. Ed. was selected for backside cover (Highlight article)
Cover: page Press Release: Here (Japanese, JST) ChemistryViews: Here
March, 2017: 119th Symposium on Catalysis Society of Japan at TMU (654 participants, Chairman)
HP
3rd International Conference on Molecular and Functional Catalysis (ICMFC-3, Singapore, February 2017)

“Effects of end-groups on photophysical properties of poly(9,9-di-n-octyl-fluorene-2,7-vinylene)s linked with metalloporphyrins: Synthesis and time-resolved fluorescence spectroscopy”
Macromolecules http://pubs.acs.org/doi/abs/10.1021/acs.macromol.7b00047
February, 2017 (Bangkok, Thailand): Pure and Applied Chemistry International Conference 2017 (PACCON2017)
February, 2017 (Bangkok, Thailand): TMU and PetroMat Joint Mini Symposium on Catalysis and Advanced Materials 2017 (Chulalongkorn University); Thammasat University; Mahidol University.

“Synthesis of (adamantylimido)vanadium(V) dimethyl complex containing (2-anilidomethyl)pyridine ligand and selected reactions: Exploring the oxidation state of the catalytically active species in ethylene dimerization” Organometallics http://pubs.acs.org/doi/abs/10.1021/acs.organomet.6b00727 (Open Access)
“ADMET Polymerization: Greener method for synthesis of end-functionalized poly(arylene vinylene)s” Green and Sustainable Chemistry (Perspective, Mini review, Open Access) https://doi.org/10.4236/gsc.2017.71001
“Synthesis of vanadium-alkylidene complexes and their use as catalysts for ring opening metathesis polymerization” Perspective in Dalton Trans. http://pubs.rsc.org/en/content/articlelanding/2016/dt/c6dt03757g#!divAbstract Cover http://pubs.rsc.org/en/content/articlepdf/2017/dt/c7dt90005h?page=search
10th International Symposium on Catalysis and Fine Chemicals 2016 (C&FC2016, Taipei, November 2016)
International Vanadium Symposium: Chemistry, Biological Chemistry, & Toxicology (V10, Taipei, November 2016)
National Taiwan University of Science and Technology (Taipei)


“Design of efficient molecular catalysts for synthesis of cyclic olefin copolymers (COC) by copolymerization of ethylene, α-olefins with norbornene, tetracyclododecene” Mini Review in Catalysts http://www.mdpi.com/2073-4344/6/11/175
“Cross metathesis of methyl oleate (MO) with terminal, internal olefins by ruthenium catalysts: Factors affecting the efficient MO conversion and the selectivity” Joint Research with UKM (Malaysia) RSC Advances http://pubs.rsc.org/en/content/articlepdf/2016/ra/c6ra24200f
August, 2017: University of Science and Technology of China (Hefei, China); Donghua University (Shanghai, China); Shanghai Institute of Organic Chemistry (SIOC), Chinese Academy of Sciences (Shanghai, China); East China University of Science and Technology (Shanghai, China)
“Ring-opening metathesis polymerization of cyclic olefins by (arylimido)vanadium(V)-alkylidenes: Highly active, thermally robust cis specific polymerization” J. Am. Chem. Soc. http://dx.doi.org/10.1021/jacs.6b06330
“Synthesis of (imido)niobium(V)-alkylidene complexes that exhibit high catalytic activities for metathesis polymerization of cyclic olefins and internal alkynes” Organometallics (Commun.) http://pubs.acs.org/doi/abs/10.1021/acs.organomet.6b00560
International Symposium on Pure and Applied Chemistry 2016 (ISPAC2016, Kuching, Sarawak, Malaysia, August, 2016).
“Synthesis and structural analysis of niobium(V) complexes containing amine triphenolate ligands of the type, [NbCl(X)(O-2,4-R2C6H2-6-CH2)3N] (R = Me, tBu; X = Cl, CF3SO3), and their use in catalysis for ethylene polymerization” Polyhedron (Special issue, Tridentate and tetradentate tripodal ligands, invited submission) http://dx.doi.org/10.1016/j.poly.2016.08.012
HTPM-9 symposium (Zengzhou, China, July 2016)

PERCH-CIC Congress IX (Pattaya, Thailand, June 2016) http://www.perch-cic-congress.org/
New laboratory picture (June, 2016)

“One-pot synthesis of end-functionalized conjugated polymers by combined acyclic diene metathesis (ADMET) polymerization with Wittig-type coupling” J. Jpn. Petrol. Inst. (Invited submission, special issue).
“Efficient norbornene (NBE) incorporation in ethylene/NBE copolymerization by half-titanocene catalysts containing chlorinated aryloxo ligands” Organometallics http://pubs.acs.org/doi/abs/10.1021/acs.organomet.6b00242
“Synthesis of (imido)vanadium(V) dichloride complexes containing anionic N-heterocyclic carbenes that contain a weakly coordinating borate moiety: New MAO-free ethylene polymerization catalysts,” Organometallics http://pubs.acs.org/doi/abs/10.1021/acs.organomet.6b00200 Most Read Article Igarashi OM No3
“Efficient synthesis of cyclic olefin copolymers with high glass transition temperatures by ethylene copolymerization with tetracyclododecene (TCD) using (tert-BuC5H4)TiCl2(N=CtBu2) – MAO catalyst,” J. Polym. Sci. Part A: Polym. Chem. (cover). http://onlinelibrary.wiley.com/doi/10.1002/pola.28144/full Cover http://onlinelibrary.wiley.com/doi/10.1002/pola.28214/abstract
“Synthesis and Structural Analysis of Zr–Al Heterobimetallic Complexes, [ZrX{(O-2,4-tBu2C6H2-6-CH2)3(μ2-O-2,4-tBu2-C6H2-6-CH2)}N][R2Al(μ2-OiPr)] [X = Cl, Et, iBu; R = Me, Et, iBu]. Unique Reactivity of the iBu Complex,” Organometallics http://pubs.acs.org/doi/abs/10.1021/acs.organomet.5b01027
“Efficient introduction of aromatic vinyl group by incorporation of divinylbiphenyl, p-divinylbenzene in syndiospecific styrene polymerization using aryloxo-modified half-titanocene catalysts,” J. Polym. Sci. Part A: Polym. Chem. (cover) http://onlinelibrary.wiley.com/doi/10.1002/pola.28091/full Cover http://onlinelibrary.wiley.com/doi/10.1002/pola.28091/abstract
“Synthesis of ultrahigh molecular weight polymers by homopolymerisation of higher α-olefins catalysed by aryloxo-modified half-titanocenes,” RSC Advances http://pubs.rsc.org/en/content/articlelanding/2016/ra/c5ra27797c#!divAbstract
PACCON2016 (Bangkok, February 2016)

“Catalytic one-pot synthesis of end-functionalized poly(9,9’-di-n-octyl-fluorene vinylene)s by acyclic diene metathesis (ADMET) polymerization using ruthenium-carbene catalysts” Macromolecules http://pubs.acs.org/doi/abs/10.1021/acs.macromol.5b02287
“Copolymerizations of norbornene, tetracyclododecene with α-olefins by half-titanocene catalysts: Efficient synthesis of highly transparent, thermal resistance polymers,” Macromolecules http://pubs.acs.org/doi/abs/10.1021/acs.macromol.5b02176
Pacifichem 2015 (Hawaii, U.S.A., December 2015)

8th AUN/SEED-Net Regional Conference on Chemical Engineering, Chemical Innovation for a Progress in ASEAN Industry and Society (Hanoi, November 2015)

Asian Polyolefin Workshop 2015 (APO2015, Tokyo Metropolitan University, November 2015) http://www.comp.tmu.ac.jp/apo2015/index.html

Tsinghua University and Renmin University of China (Beijing, China, October 2015)
ACS Advances in Polyolefins 2015 (Santa Rosa, CA, USA, September 2015)

“Time-resolved fluorescence spectra in the end-functionalized conjugated triblock copolymers consisting of poly(fluorene vinylene) and oligo(phenylene vinylene): Proposal of dynamical distortion in the excited state,” Macromolecules http://pubs.acs.org/doi/abs/10.1021/acs.macromol.5b01084
250th ACS National Meeting (Boston, U.S.A., August 2015)

“Synthesis of well-defined oligo(2,5-dialkoxy-1,4-phenylene vinylene)s with chiral end groups: Unique helical aggregations induced by the chiral chain ends,” Chem. Eur. J. Commun. http://onlinelibrary.wiley.com/doi/10.1002/chem.201503158/abstract
Department of Chemistry, Thammasat University (Bangkok, Thailand, August 2015)

Backside cover, Dalton Trans., 44(38), 16728–16736 (2015). DOI: 10.1039/C5DT02726H http://pubs.rsc.org/en/content/articlepdf/2015/dt/c5dt90171e
“Synthesis and structural analysis of tungsten-carbonyl dimers bridged with oligo(2,5-dialkoxy-1,4-phenylene vinylene)s through pyridine coordination,” Dalton Trans. http://pubs.rsc.org/en/content/articlelanding/2015/dt/c5dt02726h
21th International Symposium on Olefin Metathesis and Related Chemistry (ISOM XXI), (Graz, Austria, July 2015).

“Synthesis of half-titanocenes containing 1,3-imidazolidin-2-iminato ligands of type, Cp*TiCl2[1,3-R2(CH2N)2C=N]: Highly active catalyst precursors in ethylene (co)polymerisation,” RSC Advances http://pubs.rsc.org/en/content/articlelanding/2015/ra/c5ra11402k#!divAbstract
“Synthesis of titanium complexes containing amine triphenolate ligand of the type, [TiX{(O-2,4-R2C6H2)-6-CH2}3N], and the Ti-Al hetero-bimetallic complexes with AlMe3: Effect of terminal donor ligand in ethylene polymerization,” Organometallics http://pubs.acs.org/doi/abs/10.1021/acs.organomet.5b00303
“(Arylimido)vanadium(V)-alkylidene complexes containing fluorinated aryloxo, alkoxo ligands for fast living ring-opening metathesis polymerization (ROMP), Highly cis-specific ROMP,” J. Am. Chem. Soc. http://pubs.acs.org/doi/abs/10.1021/jacs.5b02149































































